Fetal Growth Restriction (FGR)
aka Intrauterine Growth Restriction (IUGR)
On this page
Tap a section to jump.
Overview
Fetal growth restriction (FGR), also known as intrauterine growth restriction (IUGR), is a condition in which an unborn baby (fetus) has an estimated fetal weight (EFW) or abdominal circumference (AC) below the 10th percentile for an accurately assigned gestational age. This means that the baby weighs less than or has a belly smaller than 9 out of 10 babies of the same gestational age. The most common causes of FGR are suboptimal perfusion of the maternal fetal circulation due to maternal or placental conditions, a naturally small fetus, and congenital syndromes in the fetus.
Causes of Fetal Growth Restriction
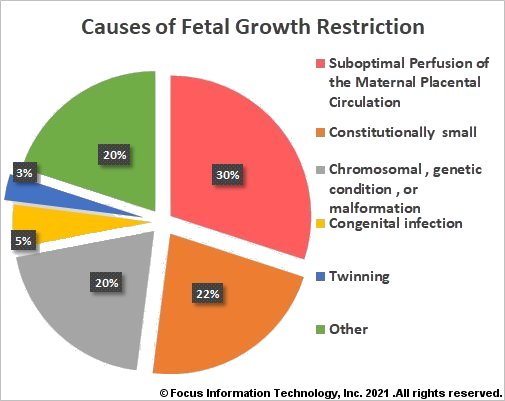
Maternal conditions that have been associated with FGR include but are not limited to hypertensive disease, antiphospholipid syndrome (APLS), diabetes with vascular disease, renal impairment, cigarette smoking, alcohol consumption, uncontrolled asthma, cystic fibrosis, cyanotic congenital heart disease, severe anemia, sickle cell anemia, β-thalassemia, and hemoglobin H disease.
Placental conditions
Preeclampsia, confined placental mosaicism, placental mesenchymal dysplasia, placental infarction and decidual vasculopathy, single umbilical artery, and velamentous cord insertion have all been associated with FGR.
Fetal conditions
Up to 22% of growth restricted fetuses are naturally small due to the size of the baby's parents, ethnic background, and the sex of the baby. About 20% of fetuses with FGR have a chromosomal (e.g. Trisomy 18, Trisomy 13, Trisomy 21, triploidy, Monosomy X) or genetic syndrome (e.g. Silver–Russell syndrome, Smith–Lemli–Opitz syndrome, Cornelia de Lange syndrome), or physical malformations such as heart defects, diaphragmatic hernia, or gastroschisis. Approximately 5% of FGR is caused by congenital infections, with cytomegalovirus (CMV) infection being the most common intrauterine infection in the United States. Multiple gestations account for 3% of all cases of FGR; up to 30% of twins may develop FGR.
Classification of FGR
CLASSIFICATION OF FGR [2]
- Early onset FGR: FGR diagnosed at less than 32 weeks
- Tends to be more severe and more likely to be associated with a congenital syndrome than late onset FGR.
- Late onset FGR: FGR diagnosed at 32 weeks or later
- Accounts for 70% to 80% of FGR cases and is typically milder than early onset FGR. Normal Doppler studies of the umbilical artery are not uncommon.
- Severe FGR: The EFW is less than 3rd percentile
Classification of FGR as symmetric or asymmetric based on the head circumference: abdominal circumference (HC/AC) ratio appears to be of limited value since the HC/AC ratio has not been found to be an independent predictor of adverse pregnancy outcomes, or of poor growth or developmental delay in growth restricted preterm newborns.
Evaluation
EVALUATION [2]
- Detailed obstetrical ultrasound for early-onset FGR
- Prenatal diagnostic testing with chromosomal microarray (CMA) for:
- Early-onset FGR OR
- FGR at any gestational age AND
- Sonographic abnormalities (fetal malformations) AND/OR
- Polyhydramnios
- Polymerase chain reaction (PCR) for cytomegalovirus (CMV) if patient has amniocentesis
- Routine TORCH serologies are not recommended in isolated FGR; test when clinically indicated.
- Evaluation may sometimes require more advanced testing methods (e.g., methylation analysis, uniparental disomy analysis, deletion/duplication analysis, sequence analysis)
Treatment
TREATMENT
Currently there are no effective treatments available for FGR. Activity restriction, and treatment with heparin or sildenafil are not recommended.
Monitoring
MONITORING
The fetus with FGR is monitored using cardiotocography (CTG) and Doppler ultrasound of the fetal umbilical arteries after viability. Routine use of ductus venosus, middle cerebral artery, or uterine artery Dopplers is not recommended for standard FGR management.
- Cardiotocography is the electronic monitoring of the fetal heart rate and uterine contraction signals. Recurrent late fetal heart rate decelerations during CTG is an indication that the fetus should be delivered.
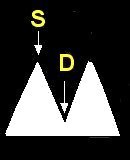
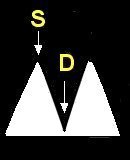
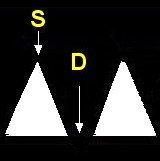
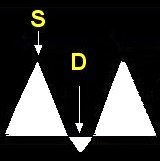
- Doppler ultrasound is used to evaluate the placenta for damage or insufficiency. Doppler ultrasound measures the velocity of the blood flow in the umbilical arteries of the fetus. The illustrations below show blood flow through the umbilical artery during contraction of the fetal heart (systolic flow). During the relaxation phase of the heartbeat there is normally continued blood flow to the placenta (end-diastolic flow). Damage or insufficiency in the placenta may be detected as decreased or absent end diastolic velocity on Doppler ultrasound.
| Normal | Decreased EDV | AEDV | REDV |
|---|---|---|---|
|
|
|
|
| Normal S/D, PI, RI ≤ 95% |
Decreased EDV S/D, PI, RI > 95% |
AEDV | REDV |
|
S = Systolic flow; Flow through the umbilical artery during contraction of the fetal heart.
D = End-diastolic flow; Continuing forward flow in the umbilical artery during the relaxation phase of the heartbeat. |
|||
Management
MANAGEMENT [2]
| FINDINGS | Frequency of UA Doppler | Frequency of Cardiotocography | Frequency of ultrasound for EFW | Delivery | |
|---|---|---|---|---|---|
| Normal Doppler | EFW ≥ 3rd % or < 10th% | Every week for 2 weeks. If stable, then every 2 to 4 weeks | Every week | Every 3 to 4 weeks | 38 0/7 to 39 0/7 weeks |
| EFW < 3rd % | Every week | Every week | Every 2 weeks | 37 0/7 weeks | |
| Decreased EDV | Every week | 1 to 2 times per week | Every 2 weeks | 37 0/7 weeks | |
| AEDV | Consider inpatient admission Corticosteroids for FLM |
2 to 3 times per week | 2 times per week if outpatient | Every 2 weeks | 33 0/7 to 34 0/7 weeks |
| REDV | Inpatient admission Corticosteroids for FLM |
1 to 2 times per day | Every 2 weeks | 30 0/7 to 32 0/7 weeks |
|
EFW = Estimated fetal weight; FLM = Fetal lung maturity
Antenatal corticosteroids are indicated if delivery is anticipated within 7 days in a woman at less than 36 6/7 weeks who has not received a previous course of antenatal corticosteroids and has no contraindications. Magnesium sulfate is recommended for neuroprotection if delivery before 32 weeks is anticipated.
Timing of Delivery for Twins with Isolated FGR [11]
References
- Giles WB, et al. Fetal umbilical artery flow velocity waveforms and placental resistance: pathological correlation. Br J Obstet Gynaecol. 1985 Jan;92(1):31-8. PMID: 3966988.
- Society for Maternal-Fetal Medicine (SMFM). Martins JG, Biggio JR, Abuhamad A. Society for Maternal-Fetal Medicine Consult Series #52: Diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2020 Oct;223(4):B2-B17. doi: 10.1016/j.ajog.2020.05.010. Epub 2020 May 12. PMID: 32407785
- American College of Obstetricians and Gynecologists (ACOG). Practice Bulletin No. 227: Fetal Growth Restriction. Obstet Gynecol. 2021 Feb;137(2):e16-e28. doi: 10.1097/AOG.0000000000004251. PMID: 33481528
- ACOG. Practice Bulletin No. 227: Fetal Growth Restriction: Correction. Obstet Gynecol. 2021 Apr;137(4):754. doi: 10.1097/AOG.0000000000004350. PMID: 33759827
- Antepartum Fetal Surveillance: ACOG Practice Bulletin, Number 229. Obstet Gynecol. 2021 Jun;137(6):e116-e127. PMID: 34011889
- Indications for Outpatient Antenatal Fetal Surveillance: ACOG Committee Opinion, Number 828. Obstet Gynecol. 2021 Jun;137(6):e177-e197. PMID: 34011892
- Longo S, Borghesi A, Tzialla C, Stronati M. IUGR and infections. Early Hum Dev. 2014 Mar;90 Suppl 1:S42-4. doi: 10.1016/S0378-3782(14)70014-3. PMID: 24709457.
- Lazzarotto T, Guerra B, Gabrielli L, Lanari M, Landini MP. Update on the prevention, diagnosis and management of cytomegalovirus infection during pregnancy. Clin Microbiol Infect. 2011 Sep;17(9):1285-93. doi: 10.1111/j.1469-0691.2011.03564.x. Epub 2011 Jun 1. PMID: 21631642.
- Medically Indicated Late-Preterm and Early-Term Deliveries: ACOG Committee Opinion Summary, Number 831 (Interim Update). Obstet Gynecol. 2021 Jul;138(1):e35-e39. doi: 10.1097/AOG.0000000000004437. PMID: 34150331
- Suhag A, Berghella V. Intrauterine Growth Restriction (IUGR): Etiology and Diagnosis. Curr Obstet Gynecol Rep 2, 102–111 (2013). DOI
- Medically indicated late-preterm and early-term deliveries. ACOG Committee Opinion No. 831. American College of Obstetricians and Gynecologists. Obstet Gynecol 2021;138:e35–9.
- Shi D, Cai L, Sun L. Genetics Etiologies Associated with Fetal Growth Restriction. Matern Fetal Med. 2022 Jul 22;4(3):206-209. PMID: 40406026
