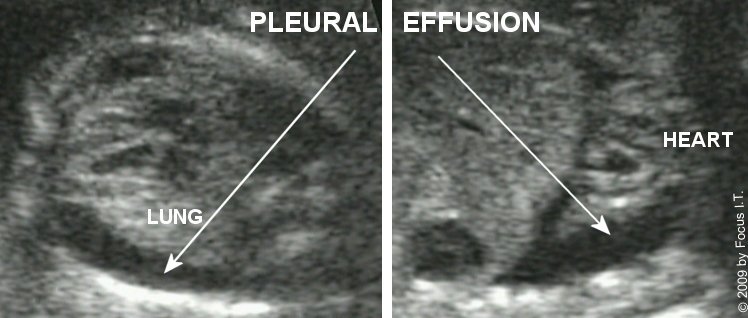
Normal thoracic anatomy & standard views
Level II thoracic evaluation focuses on heart position, lung echogenicity, diaphragm continuity, chest shape, and relationship of intrathoracic organs.
Standard thoracic views
- Four-chamber view (cross-link to cardiac module):
- Assesses cardiac position (usually left chest with apex pointing left).
- Evaluates relative lung volumes surrounding the heart.
- Transverse chest view:
- Symmetric lung fields of homogeneous, moderately echogenic appearance.
- Visualization of ribs encircling the chest.
- Sagittal and coronal views:
- Continuous, curvilinear diaphragm separating thorax and abdomen.
- Confirmation of stomach and liver position below the diaphragm.

Any deviation in organ position (e.g., stomach or liver in the thorax), lung echogenicity, or chest size should prompt detailed evaluation for CDH, CPAM, BPS, effusions, and skeletal dysplasia.