Diagnosis and Management of Varicella Infection in Pregnancy
By
Patrick Duff, M.D.
Perinatology 2010;
1:6-12
|
Clinical Case
A 27-year-old woman, gravida 3 para 2002, was exposed to chicken pox
two days ago as a result of close contact with her youngest child.
Upon questioning by her obstetrician, she indicates that she cannot
remember whether she ever had chicken pox as a young child. She also
has no memory of ever being vaccinated for chicken pox. |
|
What are the most appropriate next steps in the evaluation and management of
this patient?
|
Dr. Duff is Professor of Clinical Obstetrics and Gynecology, Division of
Maternal-Fetal Medicine at the University of Florida College of Medicine.
duffp@obgyn.ufl.edu
Overview
Varicella-zoster virus is a DNA virus. It is highly contagious;
varicella will develop in approximately 85% of susceptible household
contacts. The virus is spread from person to person via respiratory
droplets and direct contact. The incubation period of the infection is
10-14 days [1].
The incidence of varicella in pregnancy is 1-5 cases per 10,000 pregnancies. The frequency of infection is not increased among pregnant women. However, adults, including pregnant women, are much more likely than children to develop serious complications of varicella. Less than 10% of all cases of varicella occur in patients older than ten years, yet a disproportionate number of deaths occur in older individuals
[1].
The most common serious sequela of varicella in adults is pneumonia, and approximately 20% of infected adults develop this complication. The most ominous hazard of varicella is encephalitis, which occurs in
< 1% of adults[1].
Women who develop varicella during pregnancy may experience spontaneous abortion, fetal demise, and congenital anomalies. Fortunately, all these complications are unusual. In an excellent large-scale investigation, Enders et al
[2] evaluated 1373 women who experienced varicella during the first 36 weeks of pregnancy. Nine cases of congenital varicella syndrome were identified; all resulted from maternal infection in the first 20 weeks of pregnancy. The highest risk of congenital infection (2%) resulted from maternal infection in weeks 13-20 of pregnancy (7 affected infants in 351 pregnancies, 95% CI of risk 0.8-4.1%). Only two cases of congenital varicella were identified in 472 pregnancies in which maternal infection occurred before 13 weeks (observed risk 0.4%, 95% CI 0.05-1.5%). Interestingly, in 366 women who had herpes zoster infection (“shingles”) during pregnancy, there were no instances of congenital varicella (95% CI 0-1.0%).
In a smaller study, Pastuszak et al [3] compared 106 women who contracted varicella in the first 20 weeks of pregnancy with 106 age-matched, non-exposed controls. The proportion of spontaneous abortions and the mean birth weights were similar in the two groups. There were more preterm births in the women with varicella (14.3 vs 5.6%, p = 0.05). The risk of varicella embryopathy was 1.2% (95% CI 0-2.4%).
|
Diagnosis of
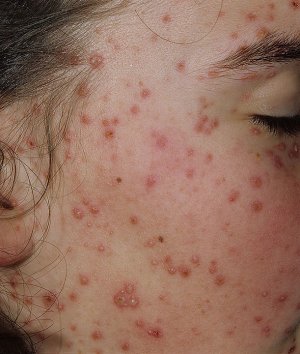
Varicella in the Mother Varicella usually causes a typical skin eruption. The lesions begin as raised red papules that progress quickly to clear vesicles. The vesicles then become cloudy pustules and, subsequently, dry to form crusted lesions. The lesions usually begin on the face and trunk and spread centripetally to the extremities. The lesions are intensely pruritic and occur in crops.
The image to the right depicts a number of varicella lesions on the face of a young woman.
Adult patients usually will have moderately severe systemic manifestations such as malaise, fatigue, and fever. Tachypnea and dyspnea
may signal the onset of varicella pneumonia which, in turn, can be
complicated by a superimposed bacterial infection. The presence of headache
and photophobia suggests the possibility of encephalitis [1].
The diagnosis of acute varicella infection can usually be established on the
basis of clinical findings alone. However, in problematic
cases, varicella-zoster serology can be performed to confirm the diagnosis. Acutely infected patients typically have positive
IgM antibody and negative IgG antibody [4 ]. |
|
 |
|
Click on image to enlarge.
Copyright © 2007
Interactive Medical Media LLC, All rights reserved. |
Diagnosis of Congenital Infection
As noted previously, the frequency of congenital malformations due to varicella infection, even in the first half of pregnancy, is = 2%
[2,3] The single best test for diagnosis of fetal injury is ultrasound examination. The principal ultrasound findings suggestive of congenital varicella infection are intrauterine growth restriction, microcephaly, ventriculomegaly, the presence of echogenic foci in the fetal liver, and circular scarring and deformities of the limbs. Chorionic villus sampling, amniocentesis, and cordocentesis
do not play an important role in the diagnosis of congenital varicella
infection [1]
Management of the Patient with Exposure to Varicella or an Acute Varicella Infection
At the time of the first prenatal appointment, the patient should be carefully questioned about prior varicella infection. Approximately 90-95% of patients of reproductive age will be immune to varicella, and they should not be at risk for second infections. If the patient has a well-documented history of natural infection, no additional testing is indicated. If she is uncertain about prior infection, a varicella-zoster IgG antibody assay should be obtained
[4]. If this test is positive, the patient can be reassured that she is immune and, therefore, not at risk for a second infection. If IgG antibody is not detected, the patient should be counseled that she is susceptible, and she should be told to avoid exposure to other individuals who may have acute varicella. She also should be counseled to avoid contact with patients who have herpes-zoster infection because she may acquire chicken pox as a result of direct contact with skin lesions in these individuals.
If a susceptible pregnant woman is exposed to someone with chicken pox, prophylactic treatment is indicated
[5]. The most well-studied method of prophylaxis is administration of varicella zoster immune globulin (VZIG). This agent is administered intramuscularly in a dose of one vial per 10 kg of actual weight, up to a maximum of five vials. Ideally, VZIG should be administered within 96 hours of exposure. Because VZIG may prolong the incubation of the virus for at least one week, a patient who receives this agent should be observed closely for possible infection for at least 28 days after receipt of VZIG.
Unfortunately, VZIG no longer is readily available in the United States from a
domestic manufacturer. The product is available only by special order from a
Canadian pharmaceutical firm,
through the U.S. distributor,
FFF Enterprises
and, thus, it may not be readily accessible to most practitioners.
If VZIG is not immediately available, clinicians should provide prophylaxis with acyclovir (800 mg orally 5 times daily for 7 days) or valacyclovir (1000 mg orally 3 times daily for seven days)[5,6] Both regimens should be comparable in effectiveness, but the former is less expensive.
If a patient develops acute varicella, with or without prophylaxis, she should be treated immediately with oral acyclovir or valacyclovir, in the doses presented previously
[5]. In a landmark investigation by Dunkle et al, [7] acyclovir reduced the duration and severity of chicken pox in normal children when therapy was initiated within 24 hours of the onset of the rash. In a subsequent randomized, placebo-controlled trial of oral acyclovir in adults, Wallace and coworkers
[8] demonstrated that treatment (800 mg five times daily for 7 days) reduced the time to crusting of lesions from 7.4 to 5.6 days (P = 0.001), reduced the maximum number of lesions by 46% (p = 0.04), and also significantly decreased the duration of fever and severity of symptoms.
If a patient develops evidence of pneumonia, encephalitis, severe disseminated infection, or if she is immunosuppressed,
she should be hospitalized and treated with intravenous acyclovir. The
appropriate dose for intravenous administration of acyclovir is 10 mg per kg
every 8 hours for 10 days. In obese patients, ideal body weight should be used
to calculate the dose of acyclovir.
Regrettably, there are no data indicating that treatment of the mother infected
with varicella will prevent congenital varicella infection. Therefore, if
evidence of fetal injury is documented, the only management options are
expectant observation vs. pregnancy termination.
Neonatal Varicella Infection
As outlined above, when maternal infection occurs early in pregnancy, the risk to the fetus is very low. However, when infection occurs in the period five days prior to, and two days after delivery, the newborn infant is at considerable risk for developing neonatal varicella. Prior to the availability of antiviral drugs such as acyclovir, 20-30% of infants delivered in this situation developed varicella, and up to 30% of affected infants died. Possible manifestations of neonatal varicella include disseminated mucocutaneous infection, visceral infection, and pneumonia
[1].
In order to prevent neonatal varicella, the newborn should be isolated from the mother until all her lesions have crusted and dried. The infant should also be treated immediately with either VZIG or antiviral chemotherapy.
Prevention of Varicella Infection
Fortunately, a highly effective vaccine for prevention of varicella now is available
[5,9]. The varicella vaccine (Varivax®) is a live virus, single-valent vaccine. The vaccine also can be administered in quadravalent form, in combination with the measles, mumps, and rubella vaccine (MMRV).
The efficacy of the varicella vaccine is 70-80% in adults and higher in
children. Children in the age range 1-12 years require one subcutaneous dose of
the vaccine. Individuals greater than 12 years of age should receive two doses
of the vaccine, separated by 4-6 weeks. Since wide-spread vaccination was begun
in the mid to late 1990s, mortality due to varicella has sharply declined [10].
Women of reproductive age who are susceptible to varicella should be offered
the varicella vaccine at the time of their annual examination or preconception
counseling appointment. Similarly, susceptible pregnant women should be offered
the vaccine immediately after delivery. Secure contraception is indicated for a
minimum of one month after the second dose of the vaccine is administered [11].
Adverse reactions to the vaccine are uncommon. The principal ones include mild
fever, inflammation and pain at the injection site, and a rash.
Contraindications to the varicella vaccine are listed in Table 1.
Table 1. Principal Contraindications to the Live Virus Varicella Vaccine
- Pregnancy
- Immunodeficiency disorder*
- High dose systemic steroid therapy
- Allergy to neomycin
- Untreated active tuberculosis
- Severe systemic illness
*Immunodeficiency is a relative contraindication to vaccination.
The vaccine has been tested and found to be safe and relatively effective in immunosuppressed
patients [12] . In immunocompromised patients, the risks and benefits of vaccination must be weighed, and each decision must be individualized.
|
Conclusion
In the clinical case presented at the beginning of this article, the patient was uncertain about immunity to varicella. Therefore, once exposure was documented, she should have a varicella-zoster IgG assay. If the IgG assay is positive, she can be reassured that she is immune. If the IgG is negative, she should be offered prophylaxis with either VZIG or acyclovir. If she develops infection despite prophylaxis, she should be treated with therapeutic doses of acyclovir for seven days. Should she develop one of the serious sequelae
of varicella, she should be hospitalized and treated with intravenous acyclovir.
Following treatment, serial ultrasound examinations should be performed to
assess for findings suggestive of congenital varicella.
Figure 1 summarizes the management algorithm for
varicella infection in pregnancy.
References
1. Chapman S, Duff P. Varicella in pregnancy. Semin Perinatol 1993;17:403-9. .PMID:8160024
2. Enders G, Miller E, Cradock-Watson, J. et al. Consequences of varicella and herpes zoster in pregnancy: prospective study of 1739 cases. Lancet 1994; 343:1547-50.
PMID: 7802767
3. Pastuszak AL, Levy M, Schick B, et al. Outcome after maternal varicella infection in the first 20 weeks of pregnancy. N Engl J Med 1994;330:901-5.
PMID: 8114861
4. McGregor JA, Mark S, Crawford GP, et al. Varicella zoster antibody testing in the care of pregnant women exposed to varicella. Am J Obstet Gynecol 1987;157:281-4.
PMID: 3039845
5. Advisory Committee on Immunization Practices. Prevention of varicella. MMWR 2007;56:1-40.
PMID: 17585291
6. Asano Y, Yoshikawa T, Suga S, et al. Postexposure prophylaxis of varicella in family contact by oral acyclovir. Pediatrics 1993:92:219-22.
PMID: 8393173
7. Dunkle LM, Arvin AM, Whitley RJ, et al. A controlled trial of acyclovir for chickenpox in normal children. N Engl J Med 1991;325:1539-44.
PMID: 1944438
8. Wallace MR, Bowler WA, Murray NB, et al. Treatment of adult varicella with oral acyclovir. A randomized, placebo-controlled trial. Ann Int Med 1992;117:358-63.
PMID: 1323943
9. Vázquez M, LaRussa PS, Gershon AA, et al. The effectiveness of the varicella vaccine in clinical practice. N Engl J Med 2001;344:955-60.
PMID: 11274621
10. Nguyen HQ, Jumaan AO, Seward JF. Decline in mortality due to varicella after implementation of varicella vaccination in the United States. N Engl J Med 2005;352:450-8.
PMID: 15689583
11. Smith WJ, Jackson LA, Watts DH, et al. Prevention of chickenpox in reproductive-age women: cost-effectivess of routine prenatal screening with postpartum vaccination of susceptibles. Obstet Gynecol 1998;92:535- 45.
PMID: 9764625
12. Asano Y, Suga S, Yoshikawa T, et al. Experience and reason: twenty year follow-up of protective immunity of the Oka Strain live varicella vaccine. Pediatrics 1994;94:524-6.
PMID: 7936864
|
|
|

